
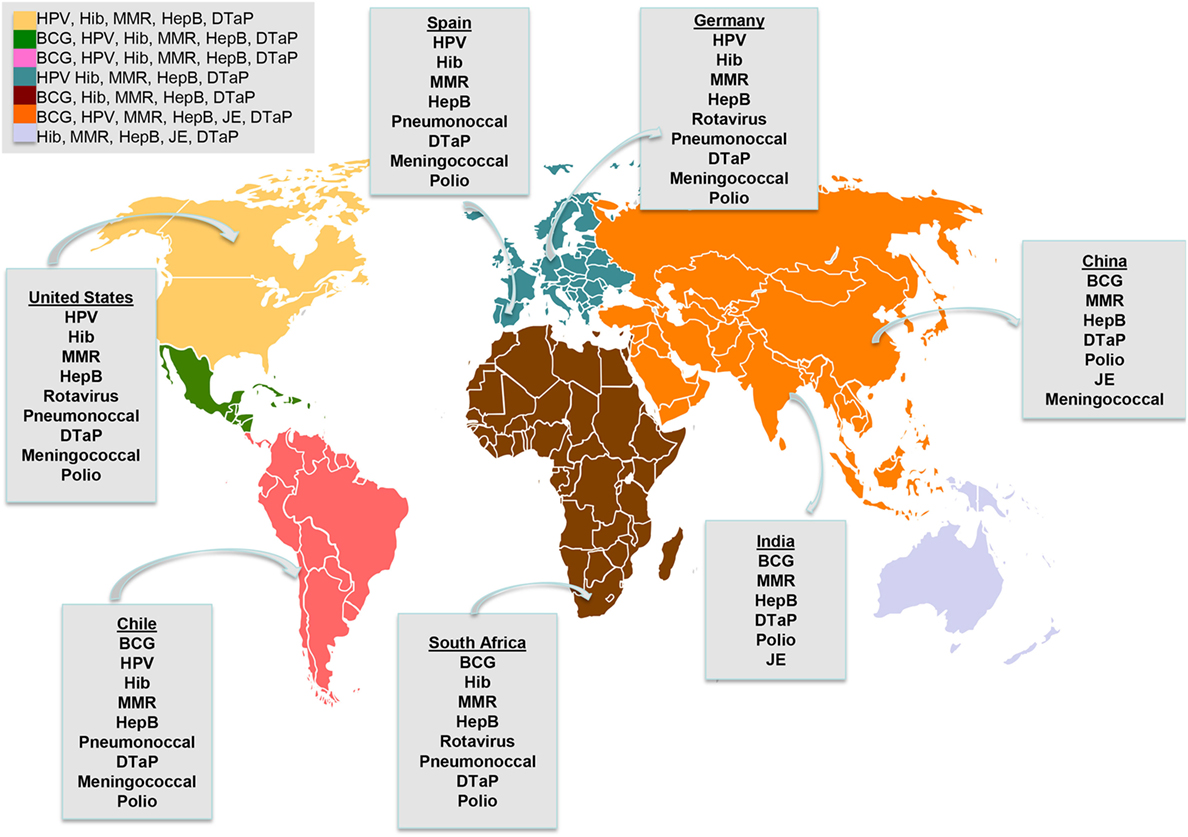
With a very large domestic market primarily for biogenerics and vaccines, China now ranks fifth worldwide in total capacity. But China, which ranks second, has 209 facilities, with about half the US average capacity/facility. The principle is that manufacturing should benefit the entire region, with regional pharmaceutical production and distribution of the vaccines by PAHO’s Revolving Fund to all countries. The White House, under pressure to increase the supply of coronavirus vaccines to poor nations, plans to invest billions of dollars to expand U.S. Much of India’s well-established biomanufacturing expertise and capacity is in vaccines production. The goal is to take advantage of existing production capacities that could contribute to manufacturing mRNA vaccines in the Americas. EU nations have criticised a call by the US to waive COVID-19 vaccine patents as a way to. The platform will foster research and incentivize the development and manufacturing of essential and strategic health technologies in the Americas, expanding manufacturing capacities to ensure access to safe, effective, and quality products, facilitating information exchange and foster cooperation between partners and countries. Africa and Latin America have 0.17 and 2 percent respectively of global production capacity. We operate one of the most sophisticated supply chain systems in the industry, with over 35 Pfizer-owned sites and over 300 suppliers globally, which provides capacity and redundancy as needed. Pfizer consistently and diligently monitors the supply of our medicines. BENGALURU, July 13 (Reuters) - Tesla (TSLA. Generate a space for discussion and exchange of experiences to identify of opportunities for regional cooperation to increase the production capacities of medicines and other essential health technologies, including vaccines, to contribute to equitable access to these products. Manufacturing and Distributing the COVID-19 Vaccine. PAHO its Member States and regional partners have renewed the efforts to improve local production capacities in the region and, at the request of Member States, PAHO launched a collaborative Platform that convene public and private stakeholders to facilitate the expansion of vaccine and other health technology research, development, and manufacturing in the Region. Information from the RFI will be provided to the COVID-19 Vaccines and Treatments for Australia – Science and Industry Technical Advisory Group.COVID-19 has shed light on the high dependence of Latin America and the Caribbean on imports of health technologies from outside the Region and the vulnerability of global supply. We are looking at over 60 responses to identify companies that can produce COVID-19 vaccines and treatments in Australia. Defence – who published a national capability audit in 2017. Data was collected on stated annual production capacity for each vaccine manufacturing facility globally.the Therapeutic Goods Administration (TGA).local and international vaccine manufacturers.
We have already collected information from: consumables, such as needles, vials and stoppers.packaging and cold chain management for handling vaccines and treatments in safe temperature ranges With current manufacturing capacity, we can make up to 5 billion vaccine doses every year, but at least 1.5 billion of those are set aside to tackle the flu.fill and finish capabilities for vaccines and treatments Our medicines supply chain is a global network that enabled us to produce and deliver 2.3 billion packs of medicines and doses of vaccines in 2022.
#Vaccine production capacity us full
To make sure we have a full understanding of current capacity, the audit includes: Our goal is to build a complete picture of our ability to produce and supply large amounts of COVID-19 vaccine and treatments within Australia. Having a complete understanding of production capacity and supply chains will help us identify facilities that could be repurposed, modified or expanded to make large quantities of a vaccine or other treatments. It will not include vaccine research and development capacity. This RFI will help to identify capacity and capability to support the manufacture, distribution and administration of COVID-19 vaccine and treatments within Australia.


 0 kommentar(er)
0 kommentar(er)
